Abstract
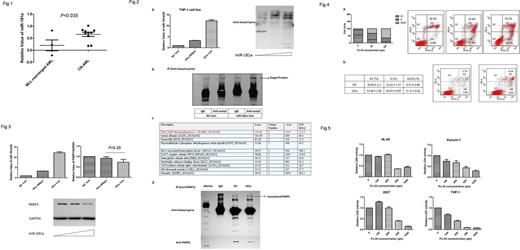
Chromosomal translocations and rearrangements involving Mixed Lineage Leukemia (MLL) gene is often associated with poor prognosis in AML. In both cytogenetically normal and abnormal AML, lower expression level of miR-181a was found to predict adverse prognosis. In our clinical study, we found that the expression level of miR-181a was significantly lower in MLL -rearranged leukemia than cytogenetically normal AML (Figure 1). To investigate the epigenetic mechanism of miR-181a, we overexpressed the miRNA in THP-1 cell line which harbors MLL-AF9 fusion gene by transfecting cells with miR-181a mimics. The effects of miR-181a overexpression on AML cell proliferation and apoptosis were studied and its effect on protein acetylation was explored in our study.
We found that miR-181a overexpression could enhance the acetylation of most proteins in THP-1 cells. After purification of acetylated proteins with immunoprecipitation, we used mass spectrum (MS) to identify the protein whose acetylation level increased the most. Poly ADP-ribose polymerase 1 (PARP1) was found (Figure 2). Overexpression of miR-181a in THP-1 cell line could down-regulate the protein level of PARP1 but not its mRNA level (Figure 3). We also observed G2/M and S phase arrest and increased apoptosis in THP-1 cells with miR-181a overexpression, which was consistent with the effects of PARP1inhibitor PJ34 (Figure 4).
In our study, we found a new pathway which explains the prognostic significance of miR-181a in MLL -rearranged AML. PARP1 protein is a critical sensor of single-strand breaks (SSBs) in base excision repair (BER) and also plays an important role in double-strand break (DSB) repair. Chromosomal instability is a main characteristic of AML and MDS, so DNA repair pathway is crucial in leukemia cells. Previous studies showed that AML driven by AML1-ETO or PML-RARα fusion oncoproteins are very sensitive to PARP1 inhibition. In our study, we found that MLL -rearranged AML was also sensitive to PARP1 inhibitor PJ34 compared to HL-60, Kasumi-1 and U937 cell lines (Figure 5). MLL -rearranged AML expressed significantly lower levels of miR-181a, which down-regulates PARP1 through increasing its acetylation level. Overexpressing miR-181a in MLL-AF9 driven THP-1 cells could cause G2/M and S phase arrest and increase apoptosis which was consistent with the effects of PJ34. PARP1 can be acetylated by p300/CBP at multiple lysine sites and then control the transcription of downstream targets by binding to its cofactor like NF-κB. However, the influence of PARP1 acetylation on protein stability has not been explored. In our study, we found that increased PARP1 acetylation was associated with reduced total protein level, the mechanism still needs to be clarified. Our study indicated that increasing miR-181a expression level and application of PARP1 inhibitors are both promising therapies in treating MLL -rearranged AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal